Buccal epithelium Expanded and Encapsulated in Scaffold-Hybrid Approach to Urethral Stricture (BEES-HAUS) procedure
Introduction:
The definitive treatment of urethral strictures involves reconstruction with additional tissue in the form of substitution urethroplasty or an end-to-end anastomotic urethroplasty. Buccal mucosal urethroplasty is an open surgical substitution urethral reconstruction. It is a major procedure with a morbidity of graft harvesting and urethral reconstruction. Although recurrence is the most worrisome complication, the procedure is associated with other complications, such as bleeding, wound infection, prolonged operative times and ejaculatory disturbances. The long-term success rates of urethroplasty surgery range from 62% to 100%, with the best results seen at high-volume centers and in the hands of experienced surgeons. The complications associated with buccal mucosal urethroplasty warrant the advent of novel techniques.
Tissue engineering technology using cell-seeded or unseeded matrices to reconstruct the urethra has been used in various studies with mixed results. The Buccal epithelium Expanded and Encapsulated in Scaffold-Hybrid Approach to Urethral Stricture (BEES-HAUS) is a tissue engineeringbased cell therapy procedure that is completely endoscopic and is a much less morbid procedure. In this procedure, autologous cultured buccal epithelial cells expanded and encapsulated in TGP scaffold are implanted at the stricture site after a wide endoscopic urethrotomy to form an epithelial layer.
Methods:
Patient Details:
The study included 6 male patients with a mean age of 41 years diagnosed with peno bulbar urethral stricture. Three patients had prior optical urethrotomy, one underwent prior buccal mucosal graft urethroplasty and two patients had no prior interventions. The AUA symptom score ranged from 16 to 22, with a mean of 19. There was no history of trauma, and the etiology was idiopathic in all patients. On physical examination, external genitalia were normal with no signs of lichen sclerosis. Patients were investigated by complete urine examination, urine culture and sensitivity, ultrasound of abdomen and pelvis, uroflowmetry, and RGU. All patients were initially subjected to small buccal mucosal biopsy under local anesthesia. The buccal epithelial cells from the biopsy were isolated and cultured in the cell culture laboratory for 10 – 12 days. Later, the patients were admitted for an endoscopic urethrotomy and implantation of cultured buccal cells embedded in the scaffold.
About the TGP scaffold:
The TGP used in the present study is a co-polymer composed of thermoresponsive polymer blocks. These thermoresponsive polymer blocks are hydrophilic at temperatures below the sol-gel transition temperature and hydrophobic at temperatures above the sol-gel transition temperature. For use in the present study, the lyophilized TGP (1 g) vial was obtained from Nichi In Biosciences, Chennai, India.
Buccal mucosal biopsy:
A small buccal mucosal graft is harvested under local anesthesia from each patient. The graft is divided into four tissue bits of 3 mm 3 each, two of the buccal mucosal tissue bits were placed in PBS and two in the TGP-based transportation cocktail with antibiotics contained in tissue transport vials. For use of serum in culture, 30 mL of the patient’s own PB was collected in each case and was transported to the laboratory in PBS at 4°C. Cells were cultured for 10 – 12 days in the laboratory.
Buccal epithelial cell culturing:
Dispase II enzyme was added to dishes containing tissue pieces & incubated at 37°C for 1 hr. Enzyme activity was then stopped by washing with DMEM containing 10% of the patient’s serum. The tissues were then treated with trypsinethylenediaminetetraacetic acid for 15 min. The cells were then cultured using a TGP-based method. Medium composed of DMEM with 10% autologous serum, insulin, penicillin-streptomycin, and amphotericin was used for culture of cells. The cells were cultured for 10–12 days. The cells proliferated and had a cobblestone appearance characteristic of epithelial cells (Fig. 1).
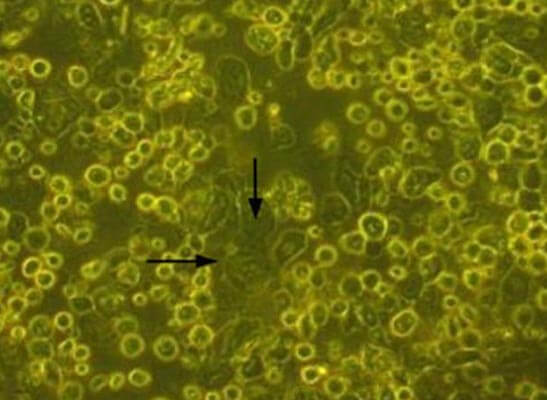
Figure 1: Cells in culture in the TGP-based method. The arrows indicate the cells with cobblestone morphology
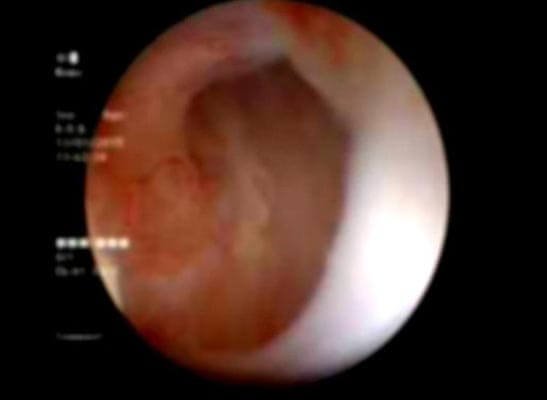
Figure 2: Follow-up Urethroscopy view at the implantation site: pink granulating tissue covering the urethrotomy site.
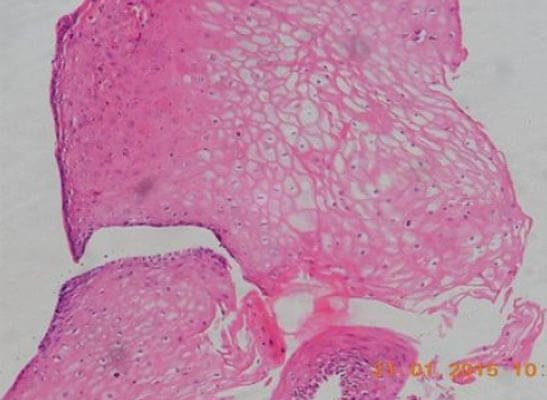
Figure 3: Photomicrograph of tissue from the implanted site of the urethra showing stratified squamous epithelium
BEES-HAUS procedure:
Cystourethroscopy was carried out under spinal anesthesia. A 0.032‐inch guide wire was passed across the stricture and using 21-Fr urethrotomy sheath and a straight cold knife, a wide dorsal urethrotomy at the stricture site was carried out from a 9 o’clock to a 3 o’clock position until petechial hemorrhages are seen. Stricture involved 2.0-3.5 cm length of bulbar/peno bulbar urethra with 1 cm of unhealthy pale mucosa on both the proximal and distal ends. Urethrotomy was extended into pale unhealthy mucosa on both the proximal and distal ends. A 14-Fr silicon Foley catheter was placed, the balloon inflated and the balloon was pulled toward the bladder neck.
Later, Urethroscopy was carried out with a 17-Fr compact Cystourethroscopy by the side of the Foley catheter to the urethrotomy site and using a 5-Fr ureteric catheter through the cystoscope, the cultured buccal epithelial cells suspended in TGP were instilled at the urethrotomy site so as to cover the entire urethrotomy site. The TGP, which was liquid at low temperature, immediately solidified at body temperature, thus fixing the cells. The catheter was left in situ for 3 weeks.
Post-treatment follow up:
The urethral catheter was removed after 3 weeks. Follow up was carried out every 6 months by symptom assessment and uroflowmetry. A check Urethroscopy was carried out at 6 months. Urethrogram was carried out at 1 year follow up.
Results:
After urethral catheter removal, all patients voided well with a good stream. There was a symptomatic improvement in all patients, and uroflow improved with a mean flow of 24 mL/s. The check Urethroscopy carried out at 6 months, 1 year and after 2 years are shown in Fig 2,3,4. Four patients were voiding well and did not require any auxiliary procedure at 3 years of follow up. Recurrence was seen in two patients at 18 months in one patient and at 2 years in the other.
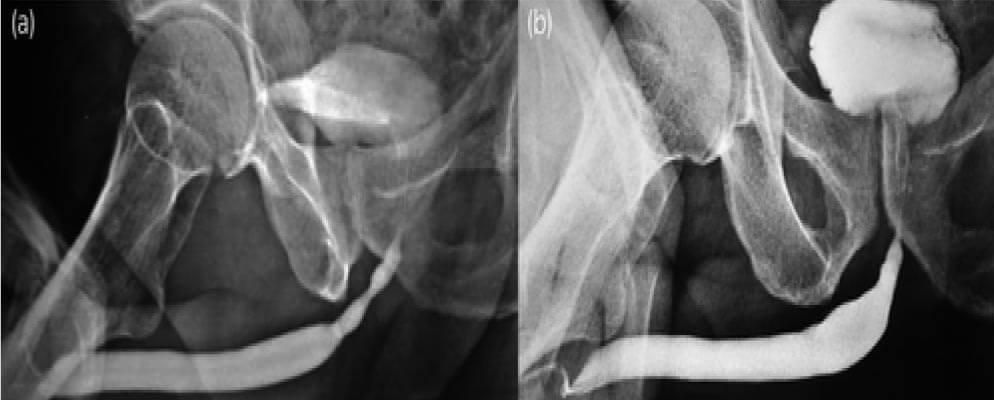
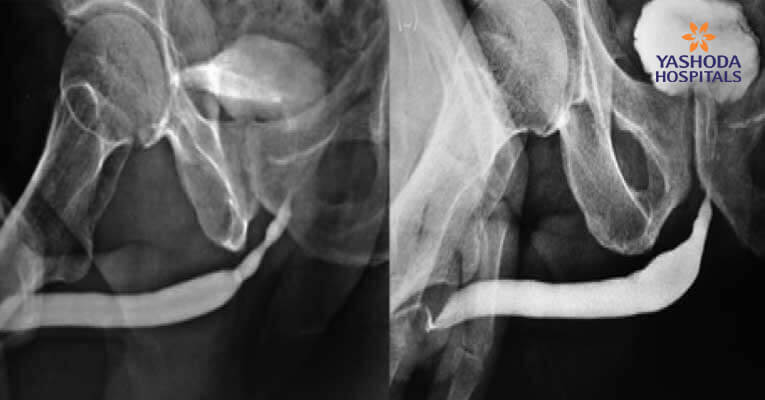
Figure 4: (a)(b) Pre-Post-BEES-HAUS procedure Urethrogram
Discussion:
The definitive treatment for urethral stricture remains one of the most challenging problems in urology. It often involves major reconstruction with additional tissue. Penile skin, scrotal skin, bladder mucosa and, recently, buccal mucosa have been used for repair of urethral defects (substitution urethroplasty). A great deal of research has been carried out to overcome this, and tissue engineering techniques could play a significant role in the reconstruction of the urethra. Hence, TGP was considered for the present study to serve as a scaffold for urethral tissue engineering. The fibrosisinhibiting nature of TGP could be of great advantage in the BEES-HAUS procedure.
Autologous buccal mucosa has been used in substitution urethroplasty for many years with acceptable results. The results of BEES-HAUS are comparable with open substitution; however, long-term follow up with a large number of patients is required. In the present study, two patients had a recurrence after 18 and 24 months of follow up, respectively. We believe that the procedure can also be used as a salvage procedure after failed buccal graft urethroplasty, as redo open surgery has significant morbidity. In long strictures and redo procedures, the donor area available is a major limitation in open substitution procedures. BEES-HAUS, an endoscopic tissue engineering-based cell therapy, has tremendous potential even in recurrent and long strictures.
In contrast to earlier published studies, where TE grafts are sutured to the native urethra by an open surgical method, our BEES-HAUS technique is entirely endoscopic with minimal morbidity. Based on the present study, we consider the use of buccal mucosal epithelial cells to be more advantageous to address the stricture of the male urethra. Our initial few cases show that novel cell-based therapy using BEES-HAUS is a promising alternative for open substitution buccal graft urethroplasty. It is possible to achieve the benefits of open substitution buccal urethroplasty with this endoscopic technique without donor site morbidity.
Abbreviations & Acronyms:
AUA: American Urological Association, BEES-HAUS: Buccal epithelium Expanded and Encapsulated in Scaffold-Hybrid Approach to Urethral Stricture, DMEM: Dulbecco’s modified Eagle’s medium, LCST: lower critical solution temperature, NIPAAm-co-BMA: N-isopropylacrylamideco-n-butyl methacrylate, PB: peripheral blood, PBS: phosphatebuffered saline, RGU: retrograde Urethrogram, TE: tissueengineered, TGP: thermoreversible gelation polymer
Acknowledgments:
The authors acknowledge Loyola ICAM College of Engineering Technology (LICET) and Loyola Institute of Frontier Energy (LIFE) for their support to his research work.
About Author –
Dr. V. Surya Prakash, Consultant Urologist, Yashoda Hospitals – Hyderabad
MS (Gen Surgery), FRCSED, M.Ch(Urology), DNB(Urology), D.Lap







 Appointment
Appointment WhatsApp
WhatsApp Call
Call More
More